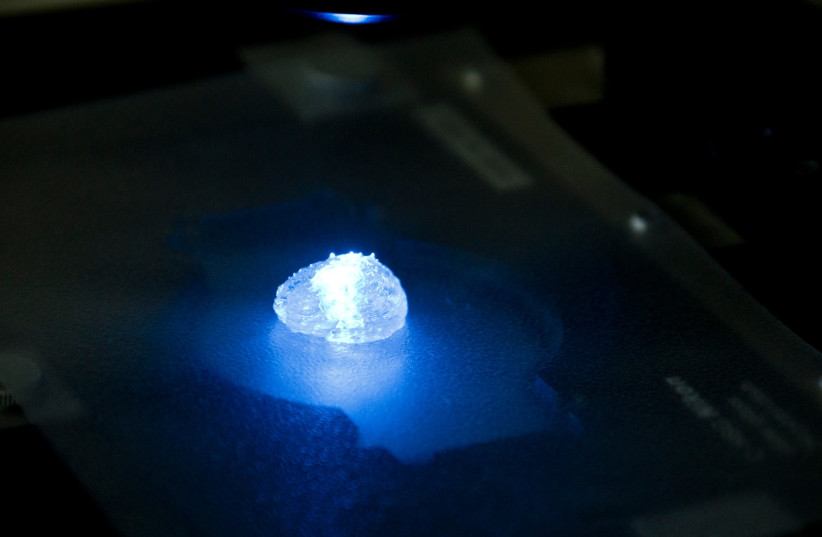
Each model is printed in a bioreactor we have designed in the lab using a hydrogel sampled and reproduced from the extracellular matrix taken from the patient, thereby simulating the tissue itself.
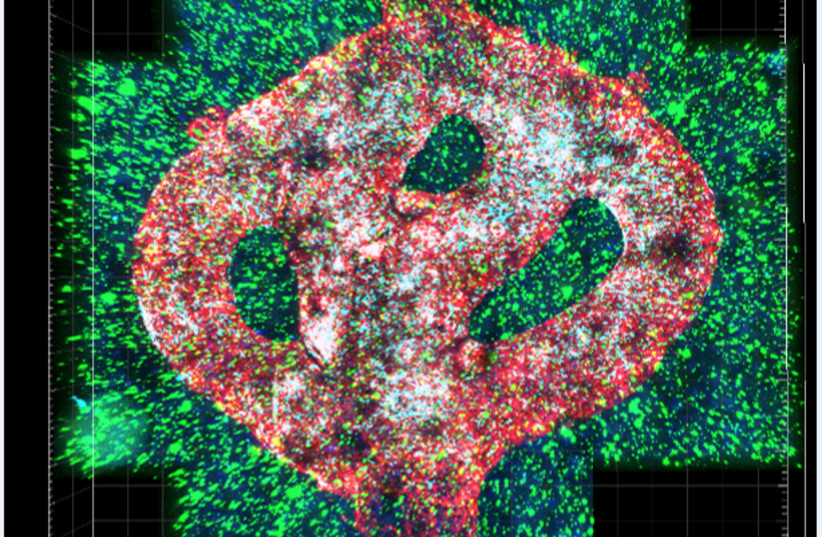
A team of Israeli researchers from Tel Aviv University has printed the world’s first viable malignant brain tumor using a 3D printer, recreating the flowing blood vessels and surrounding brain tissue.
The glioblastoma tumor is based on samples from patients taken during surgery and is surrounded by a complex system of blood vessel-like tubes through which blood cells and drugs can flow, simulating a real tumor, explained Prof. Ronit Satchi-Fainaro, who led the research.

Glioblastoma is the deadliest type of brain cancer.
The 3D model could help accelerate the discovery and development of drugs or druggable targets, and facilitate a new level of personalized medicine for patients, enabling fast and more robust prediction of the most suitable treatments.
The research was published Wednesday in the peer-reviewed Science Advances journal. It was conducted in collaboration with doctoral student Lena Neufeld. The study was funded by the Morris Kahn Foundation, the European Research Council, the Israel Cancer Research Fund, the Israel Cancer Association, the Israel Science Foundation and Check Point Software Technologies.
Until now, cancer cells were grown on 2D plastic petri dishes.
“I would put 1,000 cells each on two petri dishes and treat one with a chemotherapeutic agent,” Satchi-Fainaro said. “The next day, or three days later, I expect to see the treated cells to be reduced to 10% of the original cells, while the control will continue to multiply every day.”
When the results were good, cancer researchers would move the agent into the clinic.
“We have been testing new drugs like this for at least three or four decades – myself included,” Satchi-Fainaro explained. But she said that 90 out of 100 compounds, when they move from the lab to the clinic, don’t work.
“It’s outrageous! And it means that something is wrong. I started to wonder. I got to the point of thinking that maybe we were working with the wrong cancer model.”
What really tipped Satchi-Fainaro off was a previous study she recently completed in which her team identified a protein called P-Selectin, produced when glioblastoma cancer cells encounter cells of the brain’s immune system.
“We found that this protein is responsible for a failure in the microglia, causing them to support rather than attack the deadly cancer cells, helping the cancer spread,” she said. “However, we identified the protein in tumors removed during surgery, but not in glioblastoma cells grown on 2D plastic petri dishes in our lab.”
THE MODEL is composed of cancer cells, and also recreates the “microenvironment in the brain” – the specialized glial cells, the primary innate immune effector cells of the central nervous system and blood vessels. The vessels are connected to a tubing system through which the team can flow red and white blood cells and different drugs to the tumor model to better predict what is effective.
“Each model is printed in a bioreactor we have designed in the lab using a hydrogel sampled and reproduced from the extracellular matrix taken from the patient, thereby simulating the tissue itself.
“The physical and mechanical properties of the brain are different from those of other organs, like the skin, breast or bone. Breast tissue consists mostly of fat; bone tissue is mostly calcium. Each tissue has its own properties, which affect the behavior of cancer cells and how they respond to medications,” said Satchi-Fainaro.
She said the model really recreates the crucial mechanical properties of the tumor and the environment in which it grows, such as plasticity and elasticity.
Once the model was created, the team sought to prove why it works better than 2D plastic dishes.
“First, we tested a substance that inhibited the protein we had recently discovered, P-Selectin, in glioblastoma cell cultures grown on 2D petri dishes, and found no difference in cell division and migration between the treated cells and the control cells which received no treatment,” Satchi-Fainaro explained. “In contrast, in both animal models and in the 3D-bioprinted models, which overexpress the protein, we were able to delay the growth and invasion of glioblastoma by blocking the P-Selectin protein.”
Next, the team conducted genetic sequencing of the cancer cells grown in the 3D model and compared them to those grown in the petri dish and a patient’s brain. The experiment showed much greater resemblance between the 3D-bioprinted tumors and the patient cells, compared to the ones grown in plastic.
“Through time, the cancer cells grown on plastic hanged considerably, finally losing any resemblance to the cancer cells in the patient’s brain tumor sample,” Satchi-Fainaro said.
Finally, they measured the tumor growth rate.
“Glioblastoma is an aggressive disease partially because it is unpredictable. When the heterogeneous cancer cells are injected separately into model animals, the cancer will remain dormant in some, while in others, an active tumor will develop rapidly,” she said.
On the dish these tumors all grow at the same rate, whereas in the 3D tumor the heterogeneity is maintained.
Satchi-Fainaro said it took the team five years to create the 3D-bioprinted tumor.
“We had a lot of difficulties and challenges on the way,” she said.
But now they are hoping that this breakthrough will change cancer research in perpetuity.
“If we take a sample from a patient’s tissue, together with its extracellular matrix, we can 3D bioprint from this sample 100 tiny tumors and test many different drugs in various combinations to discover the optimal treatment for this specific tumor,” Satchi-Fainaro said.
“Alternatively, we can test numerous compounds on a 3D-bioprinted tumor and decide which is most promising for further development and investment as a potential drug.
“But perhaps the most exciting aspect is finding novel druggable target proteins and genes in cancer cells – a very difficult task when the tumor is inside the brain of a human patient or model animal.”
She said the ultimate plan is to create other tumor sites as they did for glioblastoma, such as for the brain tumors that develop in the advanced stages of lung or breast cancer. They are launching a clinical trial at Sheba Medical Center in Tel Hashomer to validate this technique. If, after three to six months, it is shown that the patient and the model respond consistently to treatments, “imagine how much time and money we will save,” Satchi-Fainaro said.
“Our innovation gives us unprecedented access, with no time limits, to 3D tumors mimicking better the clinical scenario, enabling optimal investigation.”
As reported by The Jerusalem Post
